- 1. What are the different phases of normal wound healing?
- 2. What are the main factors involved in delayed wound healing?
- 3. Which medicinal products affect wound healing?
- 4. What is the impact of topical corticosteroids on wound healing?
- 5. Is there a difference between wound healing in acute and chronic wounds?
- 6. How should you clean wounds?
- 7. What are the specific features of wound healing in children?
- 8. What are the specific features of wound healing in the elderly?
- 9. How to manage wound healing in cancer patients?
- 10. What are the specific features of wound healing in patients with burn injuries?
- 11. What dressing should be used according to the appearance of the wound?
- 12. What role do dermocosmetics play in wound healing?
- 13. Is there a role for alternative therapies in wound healing?
- 14. What advice should be given to patients to achieve an optimal scar (postoperative and post-trauma)?
- 15. What is a pathological scar and why does it develop?
- 16. How do you treat pathological scars?
- 17. How do you treat and heal a leg ulcer?
- 18. How do you treat and heal a pressure sore?
- 19. How do you treat and heal a foot wound in a diabetic patient?
- 20. What is the relationship between wound healing and mental health?
1. What are the different phases of normal wound healing?
The healing of acute traumatic or surgical skin wounds involves a complex and unique process. To understand the general principles of wound healing, it is easier to approach the topic by distinguishing the different successive phases, although keeping in mind that they overlap in time and space (Figure 1) [1, 2]. It is also important to understand that these different physiological stages are disrupted in chronic wounds subject to delayed healing.
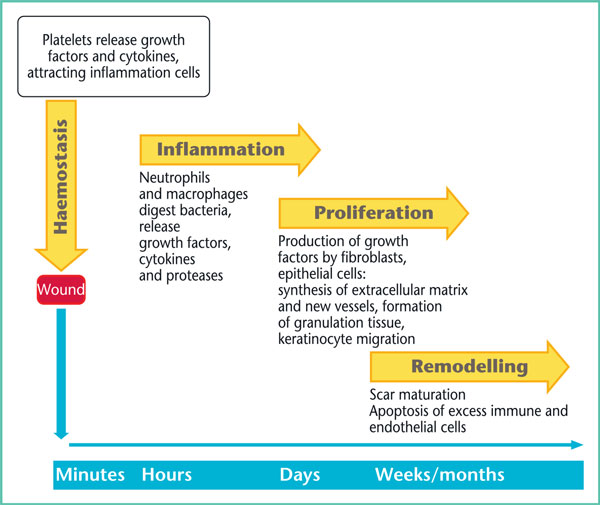
Figure 1. Diagram of physiological healing.
The first phase following wound formation is haemostasis and the formation of a temporary matrix. This phase consists of a succession of cellular and molecular mechanisms that allow not only coagulation to reduce bleeding but also involve multiple cytokines and growth factors that promote collagen synthesis, angiogenesis and even re-epithelialisation.
This is followed by an inflammation phase that begins with a primary inflammation stage, involving the recruitment of neutrophils, followed by a secondary inflammation stage during which monocytes are transformed into macrophages. In the first few days, neutrophils play a major role by combating bacterial aggression, helping to break down necrotic tissue and attracting other inflammatory cells. Approximately three days after the injury, macrophages become the primary cells of healing, through their ability to destroy cellular debris and synthesise numerous growth factors that stimulate the next proliferation phase.
Damaged tissue is rebuilt during the proliferation phase, along with re-epithelialisation and the formation of the vascular network. This crucial stage takes places during the first few weeks and results in the formation of granulation tissue. Re-epithelialisation is achieved by keratinocyte proliferation from the margins of the injury, which have the ability to migrate within the forming matrix, and by epidermal stem cells located in the “bulge” area of hair follicles (Figure 2). Physiological proliferation is achieved through a balance between degradation and synthesis of the extracellular matrix. In pathological scars, this balance becomes imbalanced in favour of degradation, in cases of delayed healing, and in favour of synthesis, in keloid and hypertrophic scars.
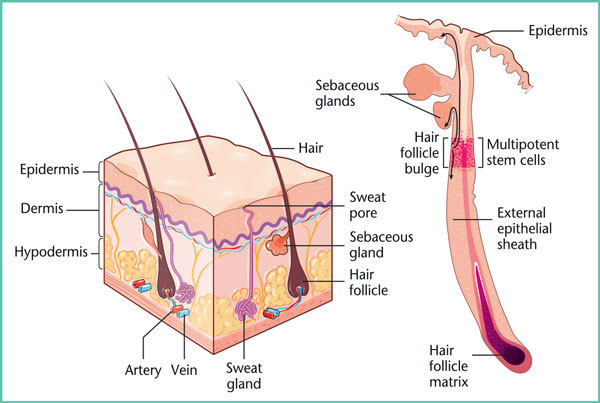
Figure 2. Skin and stem cells.
Finally, during the maturation phase a series of changes occur to components of the extracellular matrix starting about three weeks after the injury and lasting for up to two years, which is why the “final” appearance of a scar can only be assessed after this time. Collagen reorganises and remodels itself according to the traction stresses placed on the wound but it will never have the same intertwining appearance as that of uninjured skin. In addition, sweat glands and hair follicles are not reconstructed, which is why healed skin is never identical to the original skin. Finally, since melanocytic proliferation occurs at a later stage, the scar will initially appearpaler than the rest of the skin.
References
1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341: 738-46.
2. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol2007; 127: 514-25.
3. Suivi en ville des plaies chroniques : ulcère veineux de jambe, escarre, plaie du pied diabétique. Assurance maladie, octobre 2015.
4. Plaies aiguës en structure d’urgence. Référentiel de bonnes pratiques. SFMU, 2017: 32 p.
5. Sgonc R, Gruber J. Age-related aspects of cutaneous wound hea- ling: a mini-review. Gerontology 2013; 59: 159-64.
6. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen 2014; 22: 187-92.
7. Opletalová K, BlaizotX, Mourgeon B, et al. Maggot therapyfor wound debridement: a randomized multicenter trial. Arch Dermatol 2012; 148: 432-8.
8. van den Broek LJ, Limandjaja GC, Niessen FB, Gibbs S. Human hypertrophic and keloid scar models: principles, limitations and future challenges from a tissueengineering perspective. ExpDer- matol 2014; 23: 382-6.
9. Prise en charge de l’ulcère de jambe à prédominance veineuse hors pansement. Recommandations HAS, 2006.
10. La compression médicale dans les affections veineuses chroniques. Fiche de bon usage HAS, 2010.
11. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, et al. Sucrose octasulfate dressing versus control dressing in patients with neu- roischaemic diabetic foot ulcers (Explorer): an international, multi- centre, double-blind, randomised, controlled trial [published correction appears in Lancet Diabetes Endocrinol 2018]. Lancet Diabetes Endocrinol 2018; 6: 186-96.
12. Reinholz M, Poetschke J, Schwaiger H, Epple A, Ruzicka T, Gauglitz GG. The dermatology life quality index as a means to assess life quality in patients with different scar types. J Eur Acad Dermatol Venereol2015; 29: 2112-9.

